
T-Cells

What are T-Cells?
Adoptive Cell Therapy (ACT) is a treatment strategy that uses genetically modified human lymphocytes to fight diseases. The process involves using T cells that are derived from the patient, activating them outside the body and then then inducing them into the patient along with other agents to help them fight cancer. ACT has the potential to be more effective than traditional immunotherapies and vaccines because it allows for the selection and expansion of T cells that specifically target cancer cells.
There are two primary methods being used to develop ACT:
Chimeric antigen receptor (CAR) into immune cells to help them recognize and target cancer cells
T-cell Receptor (TCR)- engineered cells that can identify tumor specific epitopes presented by MHC molecules on the tumor size
The CARs assist the cells in adhering to particular proteins, called antigens, found on cancer cells (and some normal cells). They also make it easier for T cells to destroy cancer cells.
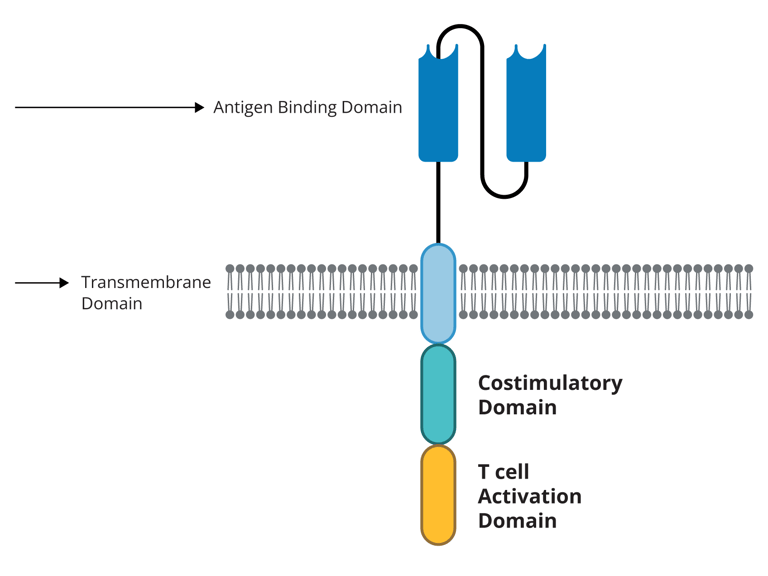
Each T cell's CAR spans the cell membrane, with a portion of the receptor located inside the cell and another outside.
Lab-made antibody fragments, or domains, make up the CAR's external portion. The receptor's capacity to identify or attach to its target antigen on tumor cells is influenced by the domains that are used.
There are "signaling" and "co-stimulatory" domains in the internal portion of every CAR. These domains send signals inside the T cells that aid in their continued proliferation throughout the body after the receptor attaches to an antigen on a tumor cell.
Modified T cells are cultured and "expanded" until they reach hundreds of millions in number. These expanded cells become the final CAR T-cell therapy product, which is then sent back to the hospital and administered to the patient through a single infusion.
Currently, the entire process, from blood collection to reinfusion, takes about 3 to 5 weeks.
After the infusion, if successful, the T cells will keep multiplying in the patient’s body and guided by their specialized receptors, target and destroy any cancer cells displaying the specific antigen on their surfaces.
Over 80% of children diagnosed with B-cell acute lymphoblastic leukemia (ALL) are cured with intensive chemotherapy, but relapsed cases lacked effective treatments for years. The approval of CAR T-cell therapy, specifically tisagenlecleucel (Kymriah), has changed this, showing long-term remissions and potential cures in children with relapsed ALL. Now a standard treatment, CAR T-cell therapies have also been approved for adults with blood cancers, such as multiple myeloma and lymphoma, offering transformative outcomes. In clinical trials, these therapies have demonstrated high success rates, with many patients remaining cancer-free for several years.
Suite 2-3, Wisma Life Care, 5, Jalan Kerinchi, Kuala Lumpur, Malaysia, 59200
+ (60)163225035 (WhatsApp)
+ (60)122963089 (WhatsApp)


